A New Era with New Medication
11/05/25 12:01
The Copaxone Era has come to an end, and the box of remaining syringes will remain untouched until I find a home for the Sharps Waste. Not sure whether my GP will take it (without charging for it) if they do charge then I’ll take it on my next Walton Centre appointment and they will take it.
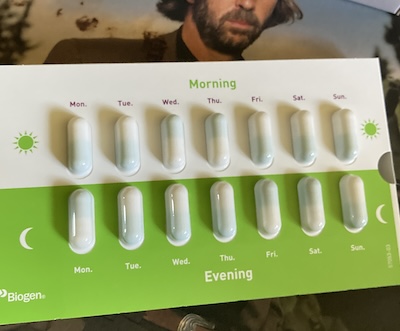
So this morning was the first time I took a dose of Tecfidera. They need to be taken with food, and that requires a change for me as I usually have just a coffee or two for breakfast. In future I may well just have Greek yoghurt and honey as my morning accompaniment to the first dimethyl fumarate capsule of the day. The medicine is taken as two doses, always with food and a minimum of four hours apart. In reality that will generally mean breakfast and evening meal. I’m not brilliant with tablets—and these are pretty big compared to any others I’ve had—but it is important that they are swallowed whole to properly deliver the dose. I’m gonna have to improve in swallowing them without coughing them out again, or damaging the coating if I don’t swallow them quickly. Twice a day forever will no doubt count as decent practice.
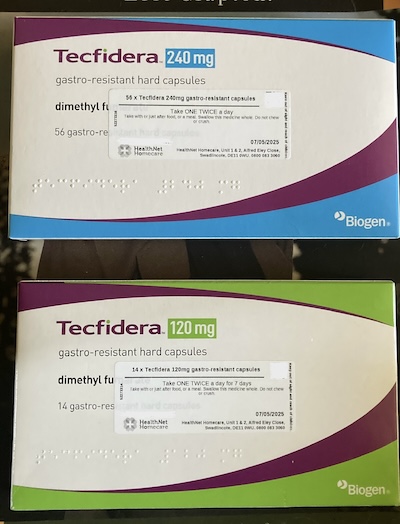
It is a week of half dose (120 mg) capsules before going on to the 240 mg dose. I haven’t opened that box, but I’m assuming (always dangerous) that the caps are the same size and not double.
I think I did get the flushing side effect a while after taking it. But my guts and beyond, which can commonly be impacted by side effects apparently, seemed unaffected. So that’s good. Will have to see how it is going forward. The booklet that comes with the drug has a notes page for keeping records and I should definitely use it for a while at least.
The medication was delivered in the afternoon. In the morning I went to the Walton Centre to meet a doctor who is undertaking a study of the effect of Tecfidera on livers (the NABS study). Apparently there is some evidence that it can positively impact on the liver and there is then some consideration being made about whether it can be used to treat patients with diseases of the liver.
I had to answer a load of questions on diet (including drink), lifestyle, and exercise. After that I was weighed, measured (height and waist), had bloods taken, and my liver was scanned (using FibroScan) . All this will provide the baseline data for the study. The doctor was actually a liver surgeon and not involved in MS. Going forward I’ll be getting scans and blood tests every three months (I need to go in every three months for the MS monitoring as well). Ideally they will undertake the tests on the same day so I wont need to go in twice.
I’m glad I volunteered for the study. More tests can only assist identifying any issues early doors for me, and more data for the study on the effects of the drug on the liver can only help others (or at least prove one way or another whether it is potentially significantly beneficial).
Incidentally the FibroScan showed my liver was in the normal range for a healthy liver with respect to fat and to stiffness. Now if I hadn’t volunteered then I wouldn’t know this. Already a benefit then. Let’s see what happens over the next three months.
So this morning was the first time I took a dose of Tecfidera. They need to be taken with food, and that requires a change for me as I usually have just a coffee or two for breakfast. In future I may well just have Greek yoghurt and honey as my morning accompaniment to the first dimethyl fumarate capsule of the day. The medicine is taken as two doses, always with food and a minimum of four hours apart. In reality that will generally mean breakfast and evening meal. I’m not brilliant with tablets—and these are pretty big compared to any others I’ve had—but it is important that they are swallowed whole to properly deliver the dose. I’m gonna have to improve in swallowing them without coughing them out again, or damaging the coating if I don’t swallow them quickly. Twice a day forever will no doubt count as decent practice.

It is a week of half dose (120 mg) capsules before going on to the 240 mg dose. I haven’t opened that box, but I’m assuming (always dangerous) that the caps are the same size and not double.
I think I did get the flushing side effect a while after taking it. But my guts and beyond, which can commonly be impacted by side effects apparently, seemed unaffected. So that’s good. Will have to see how it is going forward. The booklet that comes with the drug has a notes page for keeping records and I should definitely use it for a while at least.

The medication was delivered in the afternoon. In the morning I went to the Walton Centre to meet a doctor who is undertaking a study of the effect of Tecfidera on livers (the NABS study). Apparently there is some evidence that it can positively impact on the liver and there is then some consideration being made about whether it can be used to treat patients with diseases of the liver.
I had to answer a load of questions on diet (including drink), lifestyle, and exercise. After that I was weighed, measured (height and waist), had bloods taken, and my liver was scanned (using FibroScan) . All this will provide the baseline data for the study. The doctor was actually a liver surgeon and not involved in MS. Going forward I’ll be getting scans and blood tests every three months (I need to go in every three months for the MS monitoring as well). Ideally they will undertake the tests on the same day so I wont need to go in twice.
I’m glad I volunteered for the study. More tests can only assist identifying any issues early doors for me, and more data for the study on the effects of the drug on the liver can only help others (or at least prove one way or another whether it is potentially significantly beneficial).
Incidentally the FibroScan showed my liver was in the normal range for a healthy liver with respect to fat and to stiffness. Now if I hadn’t volunteered then I wouldn’t know this. Already a benefit then. Let’s see what happens over the next three months.
blog comments powered by Disqus